Space Crystals and the Search for a Cancer Cure: Using … – ISS National Lab
To take a shot at a “holy grail” of cancer drug discovery, researchers from Frederick National Laboratory for Cancer Research set their sights on space. Specifically, the team leveraged the International Space Station (ISS) National Laboratory to crystallize a protein linked to several of the deadliest cancers, including pancreatic, lung, and colon cancers. Gravity-driven forces can make it difficult to grow high-quality protein crystals on Earth. However, the ISS National Lab allows researchers to do something that cannot be done on Earth—remove gravity.
KRAS is the most frequently mutated member of the RAS family of genes, which produce proteins involved in the growth and death of cells. Mutations of the KRAS gene cause about 95% of pancreatic cancer. In fact, 30% to 40% of all cancers are driven by mutations in this gene. Knowing the structure of the protein encoded by the mutated KRAS gene would allow scientists to develop drugs to block the protein’s action and treat KRAS-related cancers. While scientists can crystallize proteins in their labs and use methods such as X-ray diffraction to determine the protein’s molecular structure, obtaining the complete detailed structure of the KRAS protein has been challenging.
“The drug discovery effort targeting RAS-driven cancer, especially KRAS-driven cancer, hasn’t been successful despite the last 20 to 30 years of effort,” said Dhirendra Simanshu, a researcher at Frederick National Laboratory working on the National Cancer Institute’s RAS Initiative. “So, from that perspective, this is one of the holy grails of cancer, to try to find a drug that could potentially target KRAS.”
Scientists know the general shape of the KRAS protein: a ball-shaped core with a flexible tail. Using crystallography, they have determined the detailed structure of the protein’s core, but it has been challenging to get a clear view of the tail because of its movement. So, the Frederick National Lab team developed a “molecular glue” to hold the tail in place during crystallization. However, when they crystallized the protein with the glue, they kept getting low-quality crystals. The data quality was so low that they could not resolve the protein structure with the glue to see if the tail held still enough to be visible. This was when they turned to space.
“The problem was the internal orderliness of the crystals,” said Albert Chan, who also works on the RAS Initiative at Frederick National Lab. “They were disordered, so we couldn’t get very good data to resolve the structure. Microgravity can help produce better crystals that provide higher-quality data, and we were hoping to solve our crystal problem—our poor data problem—by making use of the ISS National Lab.”
Getting a Look at the KRAS Protein
Proteins are complex molecules that drive cellular function to maintain our health. They are made of hundreds, or thousands, of amino acids arranged in a particular sequence, which determines a protein’s 3D structure and function. Genes provide cells with instructions to produce specific proteins for different purposes. However, when a gene mutates, the instructions it sends are not quite right. When cells produce proteins that do not function correctly, it can lead to disease.
The KRAS gene encodes a protein that attaches to the cell membrane and interacts with other proteins to signal cell growth. The KRAS protein switches between an active and inactive state, which determines whether cell growth is triggered. Normally, the protein is only active in short bursts. But in the mutant form, it is always in the active state—like a car with an accelerator pedal that gets stuck. The mutant protein persistently signals cell growth, which can cause several types of cancer.
Scientists could design cancer drugs that bind to the KRAS protein and block its function, but they need the detailed structure of the full protein. The core, called the G-domain, is made of 165 amino acids that form a ball-like shape. An additional 23 amino acids extend out from the core in a highly flexible tail-like structure called the hypervariable region (HVR). The HVR plays a critical role because it attaches the KRAS protein to the cell membrane, where it interacts with other proteins to signal cell growth.
Some data suggests that when the KRAS protein is first produced, the HVR wraps back and interacts with the G-domain, forming a pocket that could be targeted for drug development, explained Dwight Nissley, director of the Cancer Research Technology Program at Frederick National Lab. A drug could be designed to bond with the protein in this initial state, locking the tail in place and preventing the protein from attaching to the cell membrane. But to do this, researchers need to see the detailed structure of the HVR and how it interacts with the G-domain. Crystallography has allowed scientists to solve the detailed structure of the G-domain, but never the HVR interacting with the G-domain because it moves too much to get a clear view.
“The HVR is quite dynamic—imagine it wiggling back and forth, so it’s not caught,” Nissley said. “The HVR is there in the crystal structures, but it essentially becomes lost in the noise because there’s not one single conformation that is easy to see.”
Developing a Molecular Glue
To try to keep the HVR at the G-domain long enough for crystallization, the Frederick National Lab team developed a molecular compound that acts like glue. “We thought that with the help of this molecular glue, it might give us a little more time for the HVR to stay attached to the G-domain so we could crystallize the full-length structure,” Chan said.
In their lab, the researchers got protein crystals with the molecular glue. However, the crystal quality was not good enough to see the structure to know if the HVR was visible. It is like a photo that is so blurred that you cannot tell if it shows what you were trying to capture.
For example, say you were trying to take a photo showing the details of individual leaves on a tree, but the image was so blurry you could not even make out the tree. You would need a higher-quality photo to see if the detailed leaves could be seen or if they were moving too much to get a clear view. In the same way, the research team needed higher-quality data to see the protein structure with the glue to find out if the HVR could be seen in detail.
“The orderliness of the crystal internally is important,” Chan said. “Sometimes you can get crystals, but when you zoom in, you don’t actually have perfect, orderly crystal packing in the lattice, and so you’re going to get poor data quality.”
Bringing Two National Labs Together

To improve the quality of the data, the team turned to the ISS National Lab. For some proteins, microgravity has been shown to produce higher-quality crystals than can be achieved on Earth. This is because, in microgravity, the movement of molecules during crystallization is slower and more ordered, resulting in a more uniform crystal.
“We had heard of many cases where microgravity has helped in improving crystal quality,” Simanshu said. “We cannot change gravity in any of our experiments on the ground, but doing an experiment on the ISS allowed us to evaluate that component as well, so we jumped at the opportunity.”
Working with another national lab was one of the reasons the team was excited about the project, Nissley said. “National labs each have specific expertise, and the ISS National Lab is the only lab that’s able to do experiments in space,” he said. “It would be great to see all the national labs finding those synergies where they can help each other by working together to do something that can’t be done by one lab alone.”
Sending KRAS to Space
The team prepared samples of several mutant KRAS proteins with the molecular glue to send to the ISS for crystallization. The samples would remain on station for five weeks and then be returned for analysis. Watching their investigation launch was extraordinary, Simanshu said. “I’d seen launches on TV, but seeing it in real life and knowing we had something inside that capsule just gives it a very different feeling,” he said.
The excitement could also be felt in Maryland, where researchers at Frederick National Lab gathered in the lab’s auditorium to watch the live broadcast of the launch. “Many of us who are scientists grew up in the age of space exploration, so we have a fondness for the space program,” Nissley said. “To think that something created in this building was going to fly on a spaceflight mission was really invigorating and was a powerful experience for a lot of people at the lab.”
When the samples returned to Earth in a cargo capsule that splashed down in the Pacific Ocean, Chan was eager to bring them back to the lab for analysis. He even flew to California to retrieve the samples in person to handle the package with care. Once at the lab, Chan looked at the samples under a microscope and was excited to see several crystals. “In some of them, I noticed the crystals looked bigger with sharper edges, so they were visually better than what we had produced in our lab before,” he said.
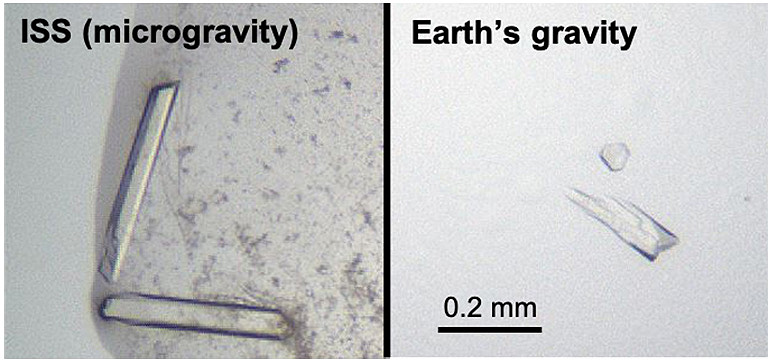
To analyze the crystals, the team worked with a third national lab—Argonne National Lab in Chicago, which houses the Advanced Photon Source that can be used for X-ray diffraction. The results showed that microgravity did solve the team’s data quality problem. The ISS-grown crystals had a 50% improvement in orderliness, and the signal-to-noise was as much as five times better using the microgravity crystals versus crystals grown on the ground.
Building Incremental Success
Using the higher-quality data from microgravity crystallization, the researchers accomplished what they could not do on Earth: resolve the structure of the KRAS protein with the molecular glue. Unfortunately, however, they could only see the detailed structure of the core. The tail of the protein was still not clearly visible. Going back to the example of the tree photo—now, the tree can be seen in the picture, but the branches were moving, and the leaves are still too blurry to see details of individual leaves.
Chan said the glue may not have been strong enough, and it could be possible to develop an improved compound that keeps the HVR attached to the G-domain for longer. Additionally, for this experiment, the samples were prepared on Earth and flash frozen for launch. Once on the ISS, the samples were thawed so the crystallization process could begin. However, it would be better for the whole process to be done in microgravity and for the team to optimize the crystallization conditions for space. This could be something the team works toward in the future.
Although the full, detailed structure of the KRAS protein continues to evade researchers, and the hunt for the holy grail of cancer continues, the team’s investigation was successful in a different way. The results provided important direction by allowing the researchers to see a structure they could not see in their lab.
It also demonstrated the potential value of microgravity crystallization for other proteins that are important drug targets but difficult to crystallize with high quality on Earth. The team’s findings showed that microgravity significantly improves the quality of certain protein crystals, resulting in data that enables structure determination not possible with crystals grown on the ground.
“I wish we could say this was the greatest discovery, but I feel, like most things in science, it’s incremental,” Nissley said. “I always tell young people that are considering going into science: You have to be ok with 90% of what you do not working. But when you build on that knowledge and then the 10% does work—that’s fantastic.”
